Costs and Benefits of Motility in E. coli
Bacteria pre-invest in motile behavior

Introduction
Microorganisms possess diverse mechanisms to regulate investment into individual cellular processes according to their environment. Bacterial responses to environmental changes range from adjustment of metabolism to induction of different stress protection mechanisms and tactic behavior. In most cases, there is an apparent qualitative benefit of investment into a cellular function under particular environmental conditions, e.g., uptake of a specific carbon source when it is available. However, how these regulatory strategies reflect the inherent trade-off between the benefit and cost of resource investment remains largely unknown, particularly for many cellular functions that are not immediately related to growth.
Swimming bacteria can typically follow various nutrient gradients in their environment by modulating the frequency of cell reorientations dependent on their swimming direction relative to the nutrient gradient. Swimming is based on rotating flagellar filaments powered by the proton motive force. A complex chemotaxis signaling pathway including a number of stimulus-specific receptors controls both chemotaxis and motility. Flagellar motility is one of the costliest bacterial behaviors. It consumes several percent of total cellular protein and energy budget, which is primarily spent for the biogenesis of flagella and powering of their rotation, respectively, and to a lesser extent for the chemotactic signaling.
When food is scarce, E. coli motility is up-regulated
Consistent with the high cost of motility, expression of flagellar genes is known to significantly reduce growth. Nevertheless, E. coli motility is up-regulated in poor carbon sources, as a part of the regulon controlled by cyclic adenosine monophosphate (cAMP) and its receptor protein (CRP).
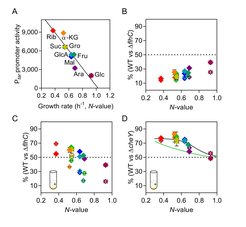
We investigated the relation between costs and benefits of motility in E. coli cells grown in a number of carbon sources of different quality (Fig. 2). The relative fitness cost of motility, i.e., growth disadvantage of motile cells under conditions where motility provides little or no benefit, was proportionally higher at lower growth rates. This increasing cost was primarily a consequence of higher investment into the biosynthesis of flagella, whereas the cost of swimming was also measurable but lower and only weakly dependent on the growth rate, and the cost the chemotactic signaling was negligible. Thus, linearly increased investment in motility at lower growth rates results in a proportionally linear increase of both the functionality and the cost of motility.
The same negative dependence on the nutritional value (N-value) of the carbon source was observed for the relative fitness benefit that is provided by chemotaxis, when chemotactic and nonchemotactic cells are cocultured in the presence of nutrient gradients. This dependence could also be seen when expression of chemotaxis and flagellar genes was largely decoupled from the growth rate, implying that increased benefit of chemotaxis at low N-values is not the consequence of increased investment but rather due to greater importance of locating additional nutrients when the primary carbon source is poor. The growth-rate dependent regulation of motility genes thus apparently ensures that investment in motility, including chemotaxis, is proportional to the fitness benefit that chemotaxis could provide when nutrient gradients are encountered in a particular carbon source.
Chemotaxis provides selective advantage even without introduced gradients
Surprisingly, the growth-rate dependent fitness benefit of chemotaxis could be observed not only when gradients of nutrients were introduced into bacterial culture, but also in self-generated gradients of the excreted secondary metabolites and of the primary carbon source. Bacterial sedimentation was the likely initial source of non-uniform distribution of bacteria in a culture under our experimental conditions, followed by chemotaxis-dependent redistribution of bacteria toward higher levels of both primary and secondary nutrients. This interplay between metabolite excretion and their chemotaxis-dependent reutilization is likely to play an important general role in microbial communities. Our work thus suggests that chemotaxis may provide a general ecological advantage in competition for reutilization of excreted metabolites. Bacteria appear to pre-invest into the motile behavior in proportion to the anticipated benefit that can be provided by chemotaxis when nutrient gradients become available in their environment, and we hypothesize that the regulation of E. coli motility has evolved under this selection.













