Regulation of motility & cell polarity
Motility in M. xanthus depends on the polar localization of motility proteins. Some of these protein localize in a stationary manner at the poles and others (such as PilB and PilT) localize dynamically to the cell poles and switch poles during a reversal. At the cellular level, these localization patterns reflect the underlying polarity of the rod-shaped M. xanthus cells with a leading and lagging cell pole.
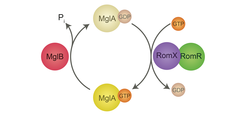
To understand regulation of motility and the underlying cell polarity, we have focused on the dynamic localization of PilB and PilT ATPases of the T4P machinery to opposite cell poles. It turns out that this localization depends on an intricate interplay between two small Ras-like GTPases and the cytoskeleton: We and others have shown that the small GTPase MglA functions as a nucleotide-dependent molecular switch to stimulate motility and that MglB represents a novel GTPase-activating protein (GAP) family and is the cognate GAP of MglA. MglA-GTP represents the active form, which stimulates motility. Lately, we have shown that MglA-GTP is targeted to the leading cell pole by the response regulator RomR, whereas MglA-GDP, the inactive form of MglA, localizes diffusely throughout the cell. MglB localizes to the lagging pole together with RomR. MglB together with RomR excludes MglA-GTP from the lagging pole by converting MglA-GTP to MglA-GDP and, thus, set up the MglA-GTP asymmetry. During reversals MglA, MglB and RomR switch poles resulting in an inversion of the leading/lagging cell polarity axis. Recently, we were able to show that MglA-GTP stimulates T4P-dependent motility by sorting PilB and PilT to opposite cell poles, i.e. MglA in the absence of MglA, PilB and PilT localize to the same cell pole.
We have also shown that the RomR response regulator interfaces with FrzZ, the output response regulator of the Frz chemosensory system, to regulate reversals. Thus, RomR serves at the interface to connect a classic bacterial signaling module (Frz) to a classic eukaryotic polarity module (MglA/MglB). Our bioinformatics analyses have shown that this modular design is paralleled by the phylogenetic distribution of the proteins suggesting an evolutionary scheme in which RomR was incorporated into the MglA/MglB module to regulate cell polarity followed by the addition of the Frz system to dynamically regulate cell polarity.
MglA is important for sorting PilB and PilT to opposite cell poles but not for polar localization per se of the two proteins. Motivated by the observation that cell polarity and motility in eukaryotic cells often depend on two or more small GTPases that act in parallel or in a cascade, we hypothesized that polar localization of PilB and PilT could involve an additional small GTPase. Indeed, we were recently able to show that the small Ras-like GTPase SofG is important for polar localization of PilB and PilT. Moreover, we have shown that SofG directly interacts with BacP, a bactofilin cytoskeletal protein, and that BacP is also required for polar localization of PilB and PilT. Using a variety of different cell imaging approaches we found that polymeric BacP localizes in both subpolar regions. SofG associates with one of these patches forming a subpolar cluster that shuttles to the pole to establish polar localization of PilB and PilT at the same pole. Following the SofG- and BacP-dependent localization of PilB and PilT to the same pole, a second event follows in which MglA sorts PilB and PilT to opposite poles to set up their correct polar localization in this way enabling T4P-dependent motility. Thus, the two small GTPases SofG and MglA function in a cascade-like manner to regulate PilB and PilT polarity. During reversals, the Frz chemosensory system causes the inversion of the leading/lagging polarity axis by inducing the relocation of MglA, MglB and RomR. Thus, three regulatory systems function in a cascade to regulate the dynamic localization of PilB and PilT.
One way to think about the MglA, MglB and RomR system is that it functions as a spatial toggle switch that is either locked in one or the other of two possible states. In response to signaling by the Frz system, the switch is flipped. This unusual system is amenable to mathematical modeling and we are intensively pursuing this approach to understand the minimal requirements for the system to work.

In our current research we are aiming at understanding how MglA, MglB and RomR localize to the cell poles and to understand in details how the Frz system “communicates” with MglA/MglB/RomR. We are also interested in understanding how MglA helps to sort PilB and PilT to opposite cell poles.
Some of our recent publications on regulation of motility & cell polarity:
Szadkowski, D., Harms, A., Carreira, L.A.M., Wigbers, M., Potapova, A., Wuichet, K., Keilberg, D., Gerland, U. & Søgaard-Andersen, L. (2019).
Spatial control of the GTPase MglA by localized RomR-RomX GEF and MglB GAP activities enables Myxococcus xanthusmotility. Nature Microbiology May 2019, doi.org/10.1038/s41564-019-0451-4
Schumacher, D. & Søgaard-Andersen, L (2017)
Regulation of cell polarity in motility and cell division in Myxococcus xanthus. Annu. Rev. Microbiol. 71, 61-78. doi: 10.1146/annurev-micro-102215-095415
McLoon, A.L., Wuichet, K., Häsler, M., Keilberg,D., Szadkowski, D. & Søgaard-Andersen, L. (2016)
MglC, a paralog of Myxococcus xanthus GTPase activating protein MglB, plays a divergent role in motility regulation. J. Bacteriol. 198, 510-520. doi: 10.1128/JB.00548-15
Treuner-Lange, A., Macia, E., Guzzo, M., Hot, E., Faure, L., Jakobczak, B., Espinosa, L., Alcor, D., Ducret, A., Keilberg, D., Castaing, J.P., Gervais, S.L., Franco, M., Søgaard-Andersen, L. & Mignot, T. (2015)
The small G-protein MglA connects to the MreB actin cytoskeleton at bacterial focal adhesions. J. Cell Biol. 210, 243-256. doi: 10.1083/jcb.201412047
Treuner-Lange, A. & Søgaard-Andersen, L. (2014)
Regulation of cell polarity in bacteria. J. Cell Biol. 206, 7-17. doi: 10.1083/jcb.201403136
Keilberg, D. & Søgaard-Andersen, L. (2014)
Regulation of bacterial cell polarity by small GTPases. Biochemistry. 53, 1899-1907. doi: 10.1021/bi500141f
Wuichet, K. & Søgaard-Andersen, L. (2014)
Evolution and diversity of the Ras superfamily of small GTPases in prokaryotes. Genome Biol. Evol. 7, 57-70. doi: 10.1093/gbe/evu264
Bulyha, I., Lindow,S., Lin, L., Bolte, K., Wuichet, K., Kahnt, J., van der Does, C., Thanbichler, M. & Søgaard-Andersen, L. (2013)
Two small GTPases act in concert with the bactofilin cytoskeleton to regulate dynamic bacterial cell polarity. Dev. Cell. 25, 119–131. doi: 10.1016/j.devcel.2013.02.017
Keilberg, D., Wuichet, K., Drescher, F. & Søgaard-Andersen, L. (2012).
A response regulator interfaces between the Frz chemosensory system and the MglA/MglB GTPase/GAP module to regulate polarity in Myxococcus xanthus. PLoS Genet. 9, e1002951. doi: 10.1371/journal.pgen.1002951
Bulyha, I., Hot, E., Huntley, S. & Søgaard-Andersen, L. (2011).
GTPases in bacterial cell polarity and signalling. Curr. Opin. Microbiol. 14, 726-733. doi: 10.1016/j.mib.2011.09.001
Miertzschke M., Koerner C., Vetter I.R., Keilberg D., Hot E., Leonardy S., Søgaard-Andersen L. & Wittinghofer A. (2011)
Mechanistic insights into bacterial polarity from structural analysis of the Ras-like G protein MglA and its cognate GAP MglB. EMBO J. 30, 4185– 4197. doi: 10.1038/emboj.2011.291
Lenz, P. & Søgaard-Andersen, L. (2011)
Temporal and spatial oscillations in bacteria. Nat. Rev. Microbiol. 9, 565-577. doi: 10.1038/nrmicro2612
Leonardy, S., Miertzschke, M., Bulyha, I., Sperling, E., Wittinghofer, A. & Søgaard-Andersen, L. (2010)
Regulation of dynamic polarity switching in bacteria by a Ras-like G-protein and its cognate GAP. EMBO J. 29, 2276–2289. doi: 10.1038/emboj.2010.114
Bulyha, I., Schmidt, C., Lenz, P., Jakovljevic, V., Höne, A., Maier, B., Hoppert, M., & Søgaard-Andersen, L. (2009)
Regulation of the type IV pili molecular machine by dynamic localization of two motor proteins. Mol. Microbiol. 74, 691–706. doi: 10.1111/j.1365-2958.2009.06891.x

