
Motility
Motility is essential for formation of both types of biofilms in M. xanthus. M. xanthus possesses two motility machineries. One motility system depends on type IV pili (T4P). The second motility machinery is for gliding motility and involves gliding motility complexes. The rod-shaped M. xanthus cells move in the direction of their long axis and have a leading cell pole and a lagging cell pole. Occasionally, cells reverse their direction of movement and during a reversal, the old leading pole becomes the new lagging cell pole. T4P assemble at the leading cell pole and during a reversal they disassemble at the old leading pole and reassemble at the new leading cell pole. In the case of the gliding motility complexes, they assemble at the leading cell pole, translocate rearward to propel cells forward, and disassemble at the lagging cell pole. During a reversal, the cell poles at which these complexes assemble and disassemble also switches. So, in total, during a reversal, the two motility machineries switch polarity.
T4P are surface structures that are found on a large number of different bacterial species and they have important function in biofilm formation, virulence and motility. T4P function depends on the assembly of a macromolecular complex consisting of 10 proteins that localize to the outer membrane, periplasm, inner membrane and cytoplasm. We have recently shown that this machinery assembles in an outward-in process starting with the PilQ secretin in the outer membrane. Moreover, using cryo-electron tomography, we were able to visualize the piliated as well as the non-piliated T4P machinery. These analyses revealed that the empty as well as the piliated T4PM in situ comprise a multi-layered structure that spans the entire cell envelope including an OM pore, three interconnected ring structures in the periplasm and cytoplasm, a cytoplasmic disc and dome, and a periplasmic stem. By visualizing a battery of assembly intermediates and T4P complexes decorated with a fluorescently-tagged protein, we systematically mapped all 10 components to these structural features revealing the overall architecture and component map of T4P machine. Finally, using available atomic structures of T4P proteins or their homologs from the type II secretion system, the component maps were informed by building pseudo-atomic hypothetical models of both piliated and non-piliated T4P machinery.
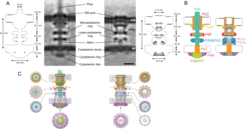
Currently, we are focusing on analyses to understand how T4P proteins are targeted to the poles. We are also focusing on analyses to understand the structure of the gliding motility complexes and how they assemble and disassemble at opposite poles.
Some of our recent publications on motility:
Bischof, L.F., Friedrich, C., Harms, A., Søgaard-Andersen, L. & Chris van der Does, C. (2016)
The type IV pilus assembly ATPase PilB of Myxococcus xanthus interacts with the inner membrane platform protein PilC and the nucleotide binding protein PilM. J. Biol. Chem. 291, 6946-6957. doi: /10.1074/JBC.M115.701284
Chang, Y.-W., Rettberg, L., Treuner-Lange, A., Iwasa, J., Søgaard-Andersen, L. & Jensen, G.J. (2016)
Architecture of the type IVa pilus machine. Science 351, aad2001. doi: 10.1126/science.aad2001
Jakobczak, B., Keilberg, D., Wuichet, K. & Søgaard-Andersen, L. (2015)
Contact- and protein transfer-dependent stimulation of assembly of the gliding motility machinery in Myxococcus xanthus. PLOS Genetics 11, e1005341. doi: 10.1371/journal.pgen.1005341
Treuner-Lange, A., Macia, E., Guzzo, M., Hot, E., Faure, L., Jakobczak, B., Espinosa, L., Alcor, D., Ducret, A., Keilberg, D., Castaing, J.P., Gervais, S.L., Franco, M., Søgaard-Andersen, L. & Mignot, T. (2015)
The small G-protein MglA connects to the MreB actin cytoskeleton at bacterial focal adhesions. J. Cell Biol. 210, 243-256. doi: 10.1083/jcb.201412047
Siewering, K., Jain, S., Friedrich, C., Webber-Birungi, M.T., Semchonok, D.A., Binzen, I., Wagner, A., Huntley, S., Kahnt, J., Klingl, A., Boekema, E.J., Søgaard-Andersen, L. & van der Does, C. (2014)
Peptidoglycan-binding protein TsaP functions in surface assembly of type IV pili. Proc. Natl. Acad. Sci. USA. 111, E953–E961.doi: 10.1073/pnas.1322889111
Friedrich, C., Bulyha, I. & Søgaard-Andersen, L. (2014)
Outside-in assembly pathway of the type IV pili system in Myxococcus xanthus. J. Bacteriol. 196, 378-390. doi: 10.1128/JB.01094-13
Bulyha, I., Lindow,S., Lin, L., Bolte, K., Wuichet, K., Kahnt, J., van der Does, C., Thanbichler, M. & Søgaard-Andersen, L. (2013)
Two small GTPases act in concert with the bactofilin cytoskeleton to regulate dynamic bacterial cell polarity. Dev. Cell. 25, 119–131 doi: 10.1016/j.devcel.2013.02.017
Søgaard-Andersen, L. (2011)
Directional intracellular trafficking in bacteria. Proc. Natl. Acad. Sci. USA 108, 7283-7284. doi: 10.1073/pnas.1104616108
Bulyha, I., Schmidt, C., Lenz, P., Jakovljevic, V., Höne, A., Maier, B., Hoppert, M., & Søgaard-Andersen, L. (2009)
Regulation of the type IV pili molecular machine by dynamic localization of two motor proteins. Mol. Microbiol. 74, 691–706. doi: 10.1111/j.1365-2958.2009.06891.x
Jakovljevic, V., Leonardy, S., Hoppert, M. & Søgaard–Andersen, L. (2008)
PilB and PilT are ATPases acting antagonistically in type IV pili function in Myxococcus xanthus. J. Bacteriol. 190, 2411–2421. doi: 10.1128/JB.01793-07
