
Intercellular communication
Over the past decades the perception of bacterial cells as autonomous individuals each following their own agenda and not interacting with each other has been replaced by the view that bacteria interact extensively both within and between species by means of intercellular signal molecules. Each of these signal molecules constitutes part of an information processing system that is constructed of four parts: The donor cell synthesizing the signal, the signal molecule, the recipient cell, and the output response. Like in any other information processing system, the signal must be tailored to the talents of the recipient. A clear example of a tailor-made signal molecule is the C-signal molecule in M. xanthus. Most intercellular signals identified in bacteria are small, i.e. with sizes < 1000 Da, freely diffusible molecules that are part of quorum sensing systems, which helps bacterial cells to assess population size. However, that is not the case for the intercellular C-signal in M. xanthus. This signal is a 17 kDa cell surface-associated protein and non-diffusible, and C-signal transmission depends on direct contacts between cells.
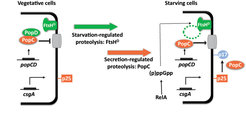
The C-signal is one of two functionally and biochemically characterized intercellular signals required for fruiting body formation. The C-signal is the intercellular signal that induces and coordinates aggregation of cells into fruiting bodies and sporulation of those cells that have accumulated inside fruiting bodies. C-signal synthesis depends on the csgA gene that encodes the full-length 25 kDa CsgA protein (p25), which is anchored in the outer membrane. p25 accumulates in vegetative cells as well as in starving cells. During starvation p25 is cleaved to a 17 kDa protein, which is also anchored in the outer membrane and exposed on the cell surface. This 17 kDa protein (p17) is the actual C-signal. So, unlike other intercellular signaling molecules identified in bacteria, the C-signal is not a small diffusible molecule but a relatively large cell-surface associated protein. Consistently, C-signal transmission depends on direct cell-cell contacts. Recently, we have figured out how p17 synthesis is restricted to starving cells by regulated proteolysis involving an FtsH protease and the secreted protease PopC.
Our current research focuses on understanding how PopC and p25 secretion is regulated and the identification of the p17 receptor.
Some of our recent publications on intercellular signaling:
Konovalova, A., Wegener-Feldbrügge, S. & Søgaard-Andersen, L. (2012).
Two intercellular signals required for fruiting body formation in Myxococcus xanthus act sequentially but non-hierarchically. Mol. Microbiol. 86, 65-81. doi: 10.1111/j.1365-2958.2012.08173.x
Konovalova, A., Löbach, S. & Søgaard-Andersen, L. (2012)
A RelA-dependent two-tiered regulated proteolysis cascade controls synthesis of a contact-dependent intercellular signal in Myxococcus xanthus. Mol. Microbiol. 84, 260-275. doi: 10.1111/j.1365-2958.2012.08020.x
Rolbetzki, A., Ammon, M., Jakovljevic, V., Konovalova, A. & Søgaard-Andersen, L. (2008)
Regulated secretion of a protease activates intercellular signaling during fruiting body formation in M. xanthus. Dev. Cell. 15, 627-634. doi: 10.1016/j.devcel.2008.08.002
Some of our recent publications on cell-cell contact-dependent activities and regulated proteolysis in bacteria:
Gómez-Santos, N., Glatter, T., Koebnik, R., Świątek-Połatyńska, M.A. & Søgaard-Andersen, L. (2019)
A TonB-dependent transporter is required for secretion of protease PopC across the bacterial outer membrane. Nature Communications vol. 10, Article number: 1360; doi: 10.1038/s41467-019-09366-9
Konovalova, A, Søgaard-Andersen, L. & Lee Kroos (2014)
Regulated proteolysis in bacterial development. FEMS Microbiol. Rev. 38, 493-522. doiI: 10.1111/1574-6976.12050
Konovalova, A. & Søgaard Andersen, L. (2011).
Close encounters: Contact-dependent interactions in bacteria. Mol. Microbiol. 91, 297-301. doi: 10.1111/j.1365-2958.2011.07711.x
