A bacterial biofilm deconstructed
Scientists at the Max Planck Institute for Terrestrial Microbiology and Princeton University have elucidated the internal organization of biofilms formed by the human pathogenic bacterium Vibrio cholerae. This work can potentially lead to the discovery of novel antibacterial and antifouling compounds.
Bacteria are ubiquitous on Earth, and they are have been found in practically every habitat ranging from a drop of water, the soil, on plants, the surface of rocks and the human skin and intestine. In all these different locations, bacteria often stick together in groups or communities called biofilms that are attached to a surface. In the biofilm, the bacteria are held together by an extracellular glue, or matrix, that the bacteria themselves produce. While biofilm formation helps to protect the bacteria, it creates immense health and industrial problems because the biofilm cells are highly resistant to antibiotics and they build-up biofouling. Although biofilms' involvement in human health and industry have driven extensive research into how these bacterial communities take hold and propagate, the internal biofilm structure is not very well understood. Scientists at the Max Plank Institute for Terrestrial Microbiology and Princeton University have now used a novel custom-built microscope to address this question. The work, which greatly improves our understanding of bacterial biofilms, is published in Proceeding of the National Academy of Sciences, USA.
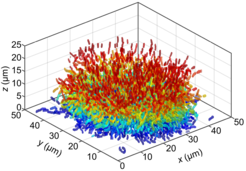
“The analysis of the detailed biofilm structure has been largely closed off to study due to limitations in microscopy and image analysis” says Knut Drescher, one of the leading authors on the paper. To overcome this problem, Knut Drescher and his collaborators built their own confocal microscope and developed novel computational methods to look at biofilms at single cell resolution. With these novel methods, they could visualize the position, size, and orientation of the thousands of individual cells that reside in Vibrio cholerae biofilms at different stages of growth.
“The investigation revealed four distinct architectural phases in Vibrio cholerae biofilm formation, each characterized by community-wide cellular reorganization. The biofilms transitioned from small groups with 1-6 cells arranged in a line, to disordered flat clusters of 20-100 cells, to disordered 3-D clusters with 200-1000 cells, and finally to highly ordered 3-D clusters with more than 2000 cells showing strong local cell alignment and a "hedgehog" pattern of global cell orientation” explains Knut Drescher.
Interestingly, the scientists also observed that transitions in biofilm architecture correlated with the total number of cells in the community, but a surprise was that cell-cell distance decreased even as the total biofilm increased in size. Furthermore, comparison of the experimental observations with stochastic biofilm models suggested that cell-cell ordering in mature biofilms does not arise passively due to cell packing. The biofilm matrix or glue presumably constrains and guides the architecture as the biofilms grow. “These findings were wholly unexpected and to test this idea we also looked at the biofilms formed by Vibrio cholerae mutants that cannot produce certain components of the matrix glue. And, indeed, we found that biofilm organization changed dramatically compared to that in normal cells” explains Knut Drescher and Carey Nadell, a postdoctoral fellow in Knut Drescher’s laboratory that was also involved in the work.
The findings outline an entirely new way to look at biofilm development, opening up a broad variety of new questions about how bacteria make biofilms. The work also provides a strong push for the marrying of concepts from molecular biology, microbiology, and physics to build a richer view of bacterial biofilms and how to fight them in the future in medicine and industry.
This work represents a collaboration of between scientists at the Max Plank Institute for Terrestrial Microbiology in Marburg, Germany and Princeton University. It also involved scientist from Massachusetts Institute of Technology and the Pasteur Institute. The study was funded supported by the Max Planck Society, the Howard Hughes Medical Institute, the National Institutes of Health USA, the National Science Foundation USA, the Human Frontier Science Program, the Alfred P. Sloan Foundation USA, the Deutsche Forschungsgemeinschaft, and the Alexander von Humboldt Foundation.
Reference:
Drescher K, Dunkel J, Nadell CD, van Teeffelen S, Grnja I, Wingreen NS, Stone HA, Bassler BL. Architectural transitions in Vibrio cholerae biofilms at single-cell resolution. Proc. Natl. Acad.Sci. USA 2016. doi: 10.1073/pnas.1601702113












