Protection of [Fe]-hydrogenase
Hydrogenase enzymes catalyze production and utilization of hydrogen gas, which is considered as a future energy carrier. Scientists at the Max Planck Institute for Terrestrial Microbiology in Marburg and a collaborator at the Max Planck Institute for Biophysics discovered that [Fe]-hydrogenase is protected by conformational change of the protein. This finding is crucial for future application of hydrogenases and for understanding the catalytic mechanism of this enzyme.
[Fe]-hydrogenase mediates reversible hydride transfer from H2 to an organic compound. This catalytic capability makes [Fe]-hydrogenase an attractive model system for designing mimetic hydrogenation catalysts; numerous mimetic compounds of the [Fe]-hydrogenase active-site have already been synthesized. One of the feature and a potential problem for application of [Fe]-hydrogenase and its mimetic catalyst is its sensitivity to light and air. Development of a protection strategy is necessary.
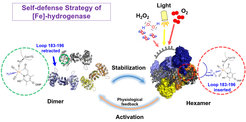
The protection of metallo-enzymes against environmental stresses is a vital process for the survival of microorganism in natural habitats, which had forced nature to develop amazingly creative solutions to maintain their enzyme active. The research group of Seigo Shima at the Max Planck Institute for Terrestrial Microbiology in Marburg together with Ulrich Ermler from Max Planck Institute of Biophysics found the protection mechanism in the crystal structure of the native [Fe]-hydrogenase from Methanothermobacter marburgensis. Based on the structural data and biochemical analysis, they found how [Fe]-hydrogenase is naturally stabilized and protected against light and oxidative stress. In the dimeric form, this enzyme is active, however, sensitive to light and air, whereas in the inactive hexameric resting form, the iron of the active site cofactor is protected by binding to an aspartate residue protruding from a loop of another dimer of the hexamer (Figure 1). This interplay between activity and stability via dynamic dimer-to-hexamer transformation and ligand exchanges is a new mode of protection. This protection mechanism can serve as an inspiration to engineer more robust hydrogenases and mimetic compounds.




![Reconstruction of a central [Fe]-Hydrogenase metallocofactor in a test tube](/1207025/original-1650443086.jpg?t=eyJ3aWR0aCI6MzYwLCJoZWlnaHQiOjI0MCwiZml0IjoiY3JvcCIsImZpbGVfZXh0ZW5zaW9uIjoianBnIiwib2JqX2lkIjoxMjA3MDI1fQ%3D%3D--1a3b0bc51a772685a00608c9f5e1a7b14a106143)






![<p>Atomic crystal structure of activated [Fe]-hydrogenase</p>](/636742/teaser-1716364267.jpg?t=eyJ3aWR0aCI6MzYwLCJoZWlnaHQiOjI0MCwiZml0IjoiY3JvcCIsImZpbGVfZXh0ZW5zaW9uIjoianBnIiwib2JqX2lkIjo2MzY3NDJ9--4e67b9a3a27597158206bf7d411649c4c854ef47)
![<p>[Mn]-hydrogenase: A new step towards redesigning hydrogenases</p>](/584881/teaser-1560940570.jpg?t=eyJ3aWR0aCI6MzYwLCJoZWlnaHQiOjI0MCwiZml0IjoiY3JvcCIsImZpbGVfZXh0ZW5zaW9uIjoianBnIiwib2JqX2lkIjo1ODQ4ODF9--dc69e8f47e0c4c4922871284f94371c72e52b9c9)