Biochemistry and evolution of natural CO2 fixing enzymes
The basis for applying, improving and re-inventing biological CO2 fixation is to understand the different mechanisms that allow enzymes to capture and convert atmospheric CO2. Nature (and in particular microorganisms) invented several different mechanisms for carbon capture and conversion – and we still keep on discovering new principles (Schada v. Borzykowski et al. Nature). Yet, even for well-studied enzymes and after years of research, many fundamental mechanisms in biological carbon fixation are not understood.
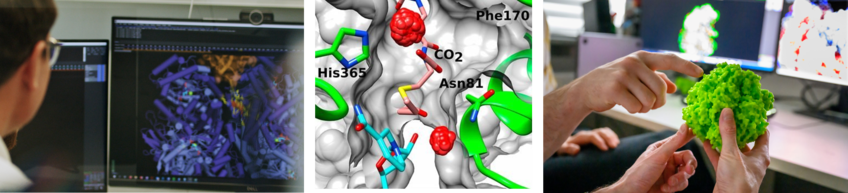
One example is Rubisco, the key enzyme in photosynthesis that captures CO2 into biomass. Despite billions of years of evolution the enzyme is (still) constrained by a trade-off between CO2-fixation activity and CO2-specificity, which limits photosynthetic carbon capture.
To understand (and eventually overcome) what constraints Rubisco’s catalysis, we have started to use ancestral reconstruction to replay crucial steps in the evolutionary history of the enzyme. We resurrect early scaffolds that just learned to fix CO2, and study Rubisco ancestors that started to loosely interact with an accessory small subunit that allowed the enzyme to immediately improve activity and specificity (Schulz et al. Science). These new insights into Nature’s No. 1 CO2-fixing enzyme provide new approaches into re-engineering Rubisco, and are enabled by cutting-edge methods like HDX, cryo-EM, NMR, as well as highly sensitive and sophisticated isotope-base enzyme assays.
Beyond Rubisco, we study another important class of CO2-fixing enzymes, enoyl-CoA reductases (ECRs) that belong to the most efficient CO2-fixing enyzmes discovered to date (Erb et al. PNAS). We have established analytical tools that allow us to resolve and dissect single steps of the catalytic cycle of ECRs to follow their catalysis almost in "slow-motion" (Rosenthal et al. Nat Chem Biol; Stoffel et al. PNAS). We also successfully used this new understanding to create new ECRs in the scaffold of ordinary reductases (Bernhardsgrütter et al. JACS).
Overall, this discovery of novel principles in catalysis provides us with the knowledge to reengineer the activity of natural CO2/C1-converting enzymes and build new-to-nature enzymes and pathways for CO2 fixation in the synthetic biology branch of our research.
Learn more about:
Engineering of new-to-nature CO2- and C1-converting enzymes
in vitro synthetic metabolic networks
Transplantation of new CO2-metabolism into natural cells
Phototrophic chassis
Design and realization of artificial organelles and cells
Return back to:
General Research Area Overview
Department Overview
Group Overview