Noisy signaling in a bacterial network
Cells being small, their signalling protein networks, on which they rely to monitor environmental conditions, are subject to strong noise, influencing the perception and thus cell behaviour. Scientists at the Max Planck Institute for Terrestrial Microbiology in Marburg and their collaborator from the Racah Institute for Physics have developed an experimental setup to follow the activity of a sensory pathway in individual Escherichia coli cells over time. They showed how noisy enzymatic reactions and thermal noise amplified by cooperative sensory arrays of receptors generate temporal fluctuations in bacterial signalling thus inducing behavioural variability in this bacterium. Their results appeared in eLife on December 12. 2017.
The chemotaxis pathway of the model bacterium Escherichia coli enables it to perceive changes in environmental conditions (concentration of nutrients and poisons, temperature, etc.) while it swims, and to modulate its swimming behaviour – alternating straight runs and reorientations – accordingly, to navigate towards more favourable conditions. For more than a decade, it has been observed that, even in homogeneous conditions, the swimming behaviour would fluctuate in time for a given bacterium, the cell alternating periods of very long straight runs with periods of very frequent directional changes. These “mood swings” are thought to maximize the chances of the bacterium to find nutrient patches when resources are scarce. It had also been predicted that these behavioural fluctuations come from fluctuations of the pathway activity, due to the noisy dynamics of two of the pathway enzymes that mediate adaptation and are present in low copy numbers in the cell.
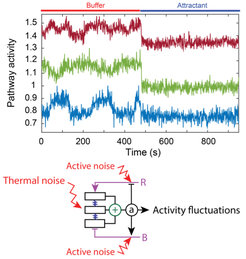
(top) Fluctuations of the activity of the chemotaxis system of E. coli, inhibited when this activity is shut down by stimulation with a strong chemical attractant. (bottom) Schematics of the pathway illustrating the two noise sources leading to these fluctuations.
Colin and co-workers applied fluorescence microscopy to measure the activity of the chemotaxis pathway in single cells on the time scale of seconds, by monitoring the activity-dependent interaction of two of its proteins. Observing directly the predicted pathway activity fluctuations, and with the help of a mathematical analysis from statistical physics, they could identify the sources of this noise. The fluctuations of the pathway activity emerge not only from the noise in the enzymatic reactions but also due to thermal agitation of the cooperative arrays of chemoreceptors. Such a large effect of the passive response of the sensory pathway to thermal noise is particularly striking, since active processes such as noisy enzyme dynamics are typically assumed to be the sources of noisy cellular behaviours. This work demonstrates that a biological system which is highly sensitive to stimulation is also inherently sensitive to noise, a relation well known for physical systems but little explored in the context of biological signalling.
This study was co-published in eLife with a second paper, “Phenotypic diversity and temporal variability in a bacterial signaling network revealed by single-cell FRET”, by J.M. Keegstra, T.S. Shimizu and coworkers of the AMOLF institute, the Netherlands. Both papers can be freely accessed online at: https://elifesciences.org.
References
Colin R., Rosazza C., Vaknin A. & Sourjik V. (2017) “Multiple sources of slow activity fluctuations in a bacterial chemosensory network.” eLife, 6:e26796 doi: 10.7554/eLife.26796
J.M. Keegstra, K. Kamino, F. Anquez, M.D. Lazova, T. Emonet and T.S. Shimizu. (2017) “Phenotypic diversity and temporal variability in a bacterial signaling network revealed by single-cell FRET.” eLife. 6:e27455 doi: 10.7554/eLife.27455












