A novel type of chemoreceptor is required for the structure and formation of cytosolic chemosensory arrays
Bacteria are experts in finding nutrients and escaping less favorable conditions. Almost all motile bacteria use the same type of sensory system to detect changes in nutrient concentrations and guide their movements toward nutrients and away from less favorable conditions. The components of this type of sensory system assemble to form large membrane-bound arrays. Scientist at the California Institute of Technology (CalTech) and the Max Planck Institute for Terrestrial Microbiology in Marburg Germany have now determined the structure of a cytoplasmic version of such a large array in the human pathogenic bacterium Vibrio cholerae. Moreover, they identified a new type of chemoreceptor with an unusual domain architecture, DosM, which is essential for formation, stability and over all structure of the cytoplasmic arrays. DosM contains two signaling domains and spans the two-layered cytoplasmic arrays.
Most motile bacteria move toward favorable environments in a process called chemotaxis. Chemotaxis depends on a chemosensory system that senses specific environmental cues and then generates an output response to regulate motility of the bacterial cells. This response enables bacteria to bias their movement away from unfavorable conditions and towards favorable conditions. Chemotaxis is mediated by large, multi-component clusters of signaling proteins, usually referred to as chemotactic signaling arrays. The molecular basis of this behavior is best understood in Escherichia coli, which encodes a single set of chemotaxis proteins that form large membrane anchored arrays. In species with more sets of chemotaxis genes and more than one type of array, an important question is whether different sets of proteins mix in different arrays or whether each cluster drives assembly of its own array.
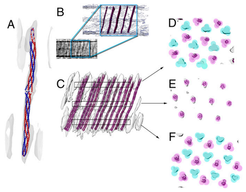
Model of the double signaling domain chemoreceptor DosM. (A) The homology model of a DosM receptor dimer (blue/red) fits into the pillar-like electron density of the subvolume average. (B) Several copies of the homology model of DosM were superimposed onto the electron density from the unaveraged tomogram, preserving both distribution and tilt angles from the fit into the subvolume average map shown in C. (C) Fitting of the homology model into the pillar-like electron densities of the subvolume average. (D–F) Cross-section of the areas marked in C, showing the locations of DosM correlating with every other trimer-of-dimer in the lattice. Each colored circle indicates a dimer; turquoise indicates dimers that are part of a trimer that is lacking DosM, and magenta indicates dimers that are DosM or are part of a DosM-containing trimer.
The bacterial human pathogen Vibrio cholerae contains three sets of chemotaxis proteins (I, II, and III). Interestingly, both membrane anchored and cytoplasmic arrays are formed in V. cholerae. The main difference between membrane-bound and cytoplasmic arrays is that in cytoplasmic chemoreceptor arrays, two layers of receptors are stacked head-to-head, sandwiched between two layers of CheA and CheW chemotaxis proteins, whereas in the membrane anchored arrays, one layer of membrane anchored receptors associate with one layer of CheA and CheW chemotaxis proteins. Using fluorescence microscopy and electron cryotomography, the research groups of Simon Ringgaard and Grant Jensen were able to show that V. cholerae’s cytoplasmic chemoreceptor array only consists of the cluster I proteins and forms independently of cluster II and III proteins. Formation of this cytoplasmic array was also found to depend on DosM, the only cytoplasmic receptor in cluster I.
Using subvolume averaging within a higher-resolution tomogram of the cytoplasmic array also revealed a unique structural element, i.e. continuous pillar-like electron densities extending between the two base plates. Homology modelling and molecular dynamics-based fitting of a single signaling domain of a DosM receptor indicated that this long, pillar-like density represents the unusual receptor DosM, whose two signaling domains point in opposite directions in trimer formation with other cytoplasmic receptors at both signaling tips (Fig.). Thus, the double signaling domain receptor protein DosM is essential for the formation and stability of cytoplasmic arrays by spanning the two-layered cytoplasmic arrays.
This work represents a collaboration of CalTech’s Division of Biology & Biological Engineering and the Max Plank Institute for Terrestrial Microbiology in Marburg, Germany. The study was funded supported by the NIH, the Howard Hughes Medical Institute, and the Max Planck Society.
Reference:
Briegel, A., Ortega, D.R, Mann, P., Kjær, A., Ringgaard, S., Jensen, G.J. Chemotaxis cluster 1 proteins form cytoplasmic arrays in Vibrio cholerae and are stabilized by a double signaling domain receptor DosM. PNAS 2016; published ahead of print August 29, 2016, doi:10.1073/pnas.1604693113
