Intracellular organization during differentiation of Vibrio parahaemolyticus in response to surface contact
Vibrio parahaemolyticus is a ubiquitous organism and a worldwide human pathogen. The bacterium possesses multiple cell types and differentiates to accommodate life under diverse conditions. It has three major life cycles; i) a planktonic free-living swimmer cell, ii) a sessile swarmer cell within microbial communities of bacteria attached to surfaces, and iii) in a host organism during infection. Growth of V. parahaemolyticus on surfaces or in viscous environments induces differentiation from the swimmer state into a swarmer cell.
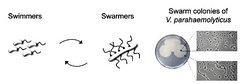
In its swimming state, V. parahaemolyticus is monotrichous, however, during swarming and in response to surface contact it differentiates into a filamentous peritrichously flagellated cell. Differentiation involves two major changes: the inhibition of cell division which results in 10-fold elongated cells (up to 30 µm in length, compared to 3 µm for swimmers), and induction of a second motility system, leading to the formation of numerous flagella positioned laterally along the cell body. Currently, little is known about how the differentiation from swimmer cells to swarmer cells is regulated. Also, it is not known how the major players controlling polarity, septation and chemotaxis are localized during this differentiation process. We are studying the mechanisms required for spatio-temporal control involved in this differentiation process to gain insights into how bacteria control protein distribution, establish cellular domains, differentiate to accommodate changes in their environment and ensure proper inheritance of macromolecular machines upon cell division.
The elongated state of swarmer cells indicates an altered regulation of cell division during differentiation of V. parahaemolyticus. To gain a complete picture of the swimmer to swarmer differentiation process, we also study the spatio-temporal dynamics of cell division and chromosome during this process. Important techniques that we use include fluorescence microscopy combined with timelapse experiments, genetics, biochemistry and bioinformatics.
Ringgaard, S., Zepeda-Rivera, M., Xiaoji, W., Schirner, K., Davis, B.M., Waldor, M.K. (2014) ParP prevents dissociation of CheA from chemotactic signaling arrays and tethers them to a polar anchor. Proc Natl Acad Sci U S A. Jan 14;111(2):E255-64
