The Stp complex as new fungicide target
All seven complex members are novel proteins without known functional domains (with the exception of the transmembrane domains in Stp5 and Stp6). They exist in all sequenced pathogenic smut species but are notably absent in other fungal species. Given the conservation in smut fungi we decided to develop the complex as new target for antifungal compounds. In collaboration with S. Sievers and her group at COMAS, we developed a yeast-based screening system in which yeast growth depends on the formation of a Stp subcomplex consisting of three of the seven complex proteins. 200,000 compounds were screened for inhibiting yeast growth only in the strain expressing the triple-subcomplex. Of 20 initial hits, three compounds also inhibited virulence of U. maydis in a concentration dependent manner. Related compounds inactive in the yeast assay were also unable to interfere with virulence in U. maydis while related compound classes affecting yeast growth negatively impacted virulence of U. maydis. The perfect correlation between inhibition of yeast growth when the Stp subcomplex is present and inhibition of virulence of U. maydis makes us confident, that we have identified antifungal compounds with a completely novel mode of action.
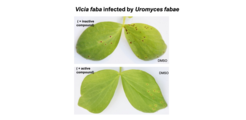
As smut fungi are not grouped among the devastating pathogens, we considered the possibility that severe agricultural threats like the rust fungi might also respond to the identified compounds. While we failed to identify members of the Stp complex by Blast searches in rust fungi, we considered that rusts, which are basidomycete fungi like U. maydis, might encode of a structurally related complex. The assertion was fueled by published EM structures by K. Mendgen and E. Kemen in which the Rtp1 translocated effector of the bean rust fungus Uromyces fabae was detected in extended structures emanating from the fungus (Kemen et al., 2013, The Plant Journal 75, 767). Amazingly, the same compounds which inhibited virulence of U. maydis suppressed pustule formation of U. fabae on broad bean leaves. This work provided the foundation for a patent application (International Patent Application No. PCT / EP2022 / 052514).