Bactofilins: a new cytoskeletal scaffold
Cytoskeletal elements have a central role in the temporal and spatial organization of cellular processes in bacteria. Among the most widely conserved cytoskeletal proteins are the tubulin homologue FtsZ, the actin homologue MreB, and intermediate filament-like proteins, which perform important roles in cell division, morphogenesis, and cell polarity.
Our group has recently identified a novel, fourth class of cytoskeletal proteins, designated bactofilins, which are widespread among bacteria. Studies in C. crescentus have shown that the two bactofilin paralogues synthesized in this organism (BacA and BacB) interact with each other and assemble into membrane-associated, polymeric sheets that are specifically localized to the old cell pole during defined stages of the cell cycle (Kühn et al., 2010). These structures serve as platforms for the assembly and polar localization of a cell wall biosynthetic enzyme (PbpC) involved in the biogenesis of stalk, an extension of the cell body characteristic of C. crescentus and many of its relatives. In vitro analysis showed that bactofilins polymerize spontaneously and in the absence of nucleotide cofactors, forming long, biochemically inert filament bundles. This behavior is reminiscent of intermediate filaments, even though there is no evolutionary or structural relationship between these two groups of proteins (Lin and Thanbichler, 2013).
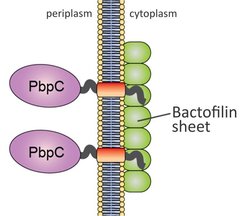
Model of C. crescentus BacAB function. Membrane-associated polymers of the bactofilins BacAB recruit the cell cell wall synthase PbpC through interaction with its N-terminal cytoplasmic tail.
Database searches showed that bactofilins are almost universially conserved among bacteria and often present in multiple paralogous copies per species. To clarify the spectrum of functions they can perform, we have set out to study the role of bactofilins in other bacteria. In particular, we are currently focusing on the delta-proteobacterium Myxococcus xanthus, an organism containing four bactofilin homologues. In collaboration with the group of Lotte Søgaard-Andersen, we have shown that three of these proteins (BacNOP) co-polymerize into extended bipolar filaments (Figure 3). These structures, on the one hand, mediate the subpolar localization of a small GTPase controlling the positioning of regulatory proteins involved in cell motility (Bulyha et al., 2013). On the other hand, they contribute to proper chromosome organization and segregation by controlling the localization of a P-loop ATPase involved in chromosome segregation (ParA) and by immobilizing the chromosomal ParB/ origin complexes in the subpolar regions of the cell (Lin et al., 2017). Collectively, these findings suggest that bactofilins serve as multifunctional polymeric scaffolds that ensure the proper arrangement of proteins within bacterial cells. Notably, work by other groups has shown that a bactofilin homolog is critical for cell shape and virulence in the human pathogen Helicobacter pylori, the causative agent of stomach ulcers. Bactofilins thus appear to have a range of critical functions that still await discovery in the future. It could be interesting to harness these structures for the development of synthetic molecular landmarks that enable the specific targeting of cellular components in artificial or heterologous cellular systems.
We are currently investigating the mechanisms regulating the assembly and positioning of bactofilin polymers. Moreover, we aim at resolving the atomic structure of bactofilin polymers and clarifying its mode of interaction with target proteins.
