Conventional methanotrophs are responsible for atmospheric methane oxidation in paddy soil
Flooded rice fields are an important source for the greenhouse gas methane, contributing about 10% to the global budget. Methane is produced by the degradation of organic matter in the absence of oxygen. Flooded soils are mostly anoxic and thus produce and emit large amounts of methane. Methane emission would even be larger if not being attenuated by methanotrophic bacteria, which oxidize much of the produced CH4 at oxic-anoxic interfaces. This oxidation operates at methane concentrations close to saturation (milimolar levels) and is achieved by conventional methanotrophs, which typically have a “low-affinity” for methane. By contrast, oxic upland soils do normally not produce methane, and act as a sink for atmospheric methane. This sink activity, which operates at the very low atmospheric methane concentrations (nanomolar level), is caused by “high-affinity” methanotrophs, which are different from the conventional methanotrophs, but have so far escaped cultivation. Hence, there is a large interest in identifying bacteria that are able to oxidize atmospheric methane.

The habitat in rice fields typically changes during the vegetation period from flooded-anoxic to drained-oxic. Wetland soils have been found to consume atmospheric CH4 during the drought season, although they do not contain “high-affinity” methanotrophs. The present study demonstrates that previous exposure to elevated methane concentrations can activate conventional methanotrophs, so that they are able to subsequently use also low atmospheric methane concentrations. This ability is gradually lost, but can be reactivated by new exposure to elevated methane. The bacteria acting as a sink for atmospheric methane were found to be conventional methanotrophs (mainly type I Methylosarcina, but also type II Methylocystis) temporarily expressing “high-affinity” for methane.
Metatranscriptome analysis of methanotrophic bacterial communities in rice field soil indicated two possible mechanisms causing expression of “high-affinity” methane oxidation. One mechanism is the specific expression of a “high-affinity” isoenzyme of the methane monooxygenase, the key enzyme of the methanotrophic bacteria. Such isoenzyme is present in many Methylocystis strains.
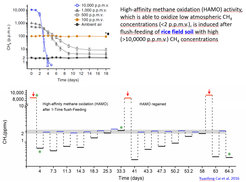
High-affinity methane oxidation (HAMO) activity, which is able to oxidize low atmospheric CH4 concentrations (flush-feeding of rice field soil with high (>10,0000 p.p.m.v.) CH4 concentrations.
The other mechanism concerns the operation of the methane monooxygenase, which uses an electron donor (e.g. NADH) to convert methane and oxygen to methanol and water. The electron donor is generated during the oxidation of methanol to carbon dioxide and thus, may be rate-limiting. Expression of “high-affinity” activity is thought to be based on the generation of endogenous storage compounds (PHB, glycogen) during flush feeding with sufficient methane. The storage compounds may serve as source of electrons for the methane monooxygenase reaction during periods of methane deprivation, thus facilitating the oxidation of low atmospheric levels of methane.
This work was supported by the National Key Basic Research Program of China (2015CB150506), the National Science Foundation of China (41401294, 31270147 and 41090281) and the Strategic Priority Research Program of the CAS (XDB15040000). We thank Ms. Dongmei Wang, Lu Lu and Gaidi Ren for their excellent technical assistance. We greatly appreciate the constructive comments of reviewers. Professor Folker Meyer at Argonne National Laboratory is gratefully acknowledged for providing bioinformatic assistance and Benli Chai at Michigan State University is acknowledged for providing database construction and helping with the subsequent screening and identification of the pmoA genes and transcripts.
Reference:
Y.Cai, Y.Zheng, P.L.E.Bodelier, R.Conrad, Z.Jia
Nature Commun., 2016, doi:10.1038/ncomms11728

